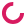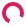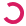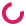The Effects of Prenatal Stress on Changes in Food, Water Consumption, Weight on Dams
Abstract
Previous research examined the influences of prenatal stress on brain development in mice, the potential of prenatal stress leading to psychiatric disorders, and the mitigating effect of docosahexaenoic acid (DHA) (Abbott et al., 2018; Matsui et al., 2018; Takeuchi et al., 2018; Jones, 2013). To study other effects of prenatal stress on mice and to ensure that behavioral effects are not driven by changes in maternal nutritional intake induced by stress, the experiment examines daily changes in food and water consumption and rate of weight gain of pregnant mice during breeding and gestation under gestational stress vs. non-stress conditions. The sample contained 22 dams, randomly assigned to the stressed group (n=10) or the non-stressed control group (n=12). The stress protocol starts on the 8th day of pregnancy. Stressed conditions include exposures to constant light, fox urine, overnight novel objects in the cage, restraint, overnight, novel noise, and multiple cage changes during the light cycle. One stressor is presented per day over a 7-day period, the sequence repeating 3 times. Food, water consumption, and weight gain are measured daily from the beginning of breeding. Dams’ daily food and water consumption and changes in weight were compared between the stressed and non-stressed groups with t-tests. The result revealed no significant differences in body weight gain, food consumption, and water consumption. This suggested that the stress protocol used in this experiment does not affect body weight gain, water consumption, and food consumption of dams during pregnancy.
Keywords: autism spectrum disorder, prenatal stress, body weight, water consumption, food consumption, mice, pregnancy
1. Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by challenges on developing typical social relations, difficulties in communicating with other people, or adherence to fixated interests and repetitive behaviors (American Psychological Association, 2013). Both genetic and environmental factors contribute to ASD, but the etiology of ASD has not been fully understood yet (Lyall et al., 2016). Past studies suggested a possible combination of maternal genetic expression, prenatal maternal stress, and maternal diet contributing to autistic-related behaviors in the offspring (Jones et al., 2010; Jones et al., 2012). Among these risk factors, both prenatal maternal stress and maternal diet are environmental risk factors related to autistic-like behaviors. Previous research has shown the relationship between prenatal stress and brain development in rodent model, the possibility of prenatal stress leading to psychiatric disorders, and the alleviation effect of docosahexaenoic acid (DHA) (Abbott et al., 2018; Matsui et al., 2018; Takeuchi et al., 2018; Jones, 2013).
Research has considered prenatal maternal stress as a risk factor of developing neuropsychiatric disorders in offspring (Abbott et al., 2018). Research found that mothers of autistic children reported higher discord and psychiatric problems during pregnancy comparing to the mothers of non-autistic children (Ward, 1990). Numerous previous studies suggested inducing stress during pregnancy may relate to increased possibility of autistic behaviors. However, other studies suggested maternal nutrition intake as a mediator for autistic behaviors among the offspring. That is, the neuropsychiatric effects on humans are due to significant nutritional deficiencies induced by the stress, not the stress itself. One experiment has shown maternal malnutrition during pregnancy may disrupt social behaviors among rodent offspring (Kinsley & Svare, 1986). Another experiment using Sprague-Dawley female rats studied the effect of nutritional stress and environmental stress during the 3rd trimester of gestation (Rhees, 1981). The result showed a longer pregnancy period for dams the environmental stress group and a significant decrease of body weight for both stress groups compared to their control group (Rhees, 1981). Another experiment using B6D2F1 breed mice studied the differences between reductions in maternal food and water intake and prenatal stress induced by physical restraint (Ward & Wainwright, 1988). Its result suggested maternal nutrition intake accounted for some deficiencies that were commonly attributed to prenatal stress (Ward & Wainwright, 1988).
In order to avoid the effect of maternal nutrition intake as mediator, the chronic variable stress the current experiment used, using constant light exposure, exposure to fox urine, overnight exposure to novel objects (marbles) in the home cage, restraint, overnight exposure to novel noise, multiple cage changes during the light cycle, are designed specifically to allow effects on cortisol without causing a reduction in weight or food intake (Harris, 2014; McEwen, 2008). Cortisol is a steroid hormone secreting by the adrenal gland in response to stress (Gartland et al., 2014, Wong et al., 2012). It is important for embryonic and fetal development (Romero-Gonzalez et al., 2018). An experiment found a positive correlation between maternal cortisol level and fetal cortisol level (Gitau et al., 1998). In addition, a longitudinal research followed 80 mothers and their babies and measured the cortisol levels from the maternal and neonatal hair (Romero-Gonzalez et al., 2018). It found an effect of prenatal stress on fetal synthesis of cortisol (Romero-Gonzalez et al., 2018). The stressors used in the current study should be stressful but will not cause a significant decrease in nutrition intake, in order to make sure the behavior changes are not driven by maternal nutrition intake.
In order to study the effects of prenatal stress without being influenced by maternal nutrition intake, it is necessary to ensure that there are no significant differences in rates of changes in body weights as well as food and water intake in the stress versus non-stress groups. Previous experiments have examined prenatal stress in relation to the food and water consumption of pregnant mice and the changes of their body weights during gestation and lactation. An animal study using Rockland-Swiss (R-S) albino female mice found reduced food and water intake of dams under heat and restraint stress (Kinsley & Svare, 1986). It also found reduced rate of body weight gain among the dams under stress condition (Kinsley & Svare, 1986). Among offspring, another animal study involving female C57/BI6 mice obtained from Jackson Laboratories used restraint stress showed no significant differences in postnatal body weight of the female offspring when they reached adult age (Gur et al., 2017). In addition, an animal experiment using B6D2F1from Charles River Breeding Laboratories found significantly lower body weight in the stressed dams but no differences in body weight of the offspring by using physical restraint as the stressor (Ward & Wainwright, 1988). Moreover, another animal study using Holtzman strain rats put half of the rodents under stress during pregnancy and found that the body weight of the male offspring was significantly lower in the prenatal stress group than the control group on both days 28 and 60, suggesting a long-lasting effect of stress on the male offspring (Cabrera et al., 1999). This study used chronic variable stress, including noise, cold, tail pinch, white noise, shaking, water deprivation, crowding, restraint, forced swim etc. (Cabrera et al., 1999).
After examining body weight, food consumption, and water consumption, brain size and behavioral measurements help determine whether prenatal stress affect brain development and autistic behaviors. For brain size, research involving pregnant C57/BI6 mice from Jackson Laboratories with stress has found that prenatal stress affected brain-derived neurotrophic factor (BDNF) in placenta (Gur et al., 2017). BDNF is a protein in the brain that supports the survival of neurons. The study found that the level of BDNF in amygdala decreased significantly among female offspring from stressed dams. This effect continued through adulthood, suggesting an extended effect of prenatal stress (Gur et al., 2017). Whereas another study using B6D2F1mice from Charles River Breeding Laboratories found significantly lighter brain weight of the pups in the stressed group compared to control group 32 days after the pups were born, there were no significant differences after 50 days (Ward & Wainwright, 1988). For anxiety-related behaviors, an experiment using a sample comprised only of female mice showed a significant increase in these kinds of behaviors among the offspring whose mother experienced prenatal stress induced by restraint (Gur et al., 2017). Another study found less motor activity and fewer social interactions as well in the group that experienced chronic variable stress compared to the non-stressed group (Cabrera et al., 1999). However, one study has found no significant differences in behaviors among the offspring in the stressed versus non-stressed groups (Ward & Wainwright, 1988).
The effect of maternal stress on mice reflects similarly in humans. To measure the correlation between maternal stress experience and the birthweight of newborns, in an adoption study, researchers analyzed 403 birth mothers and their newborns with a hierarchical regression model (Brotnow et al., 2015). The study showed support for multidimensional theoretical models of prenatal stress, which meant considering both risk factors and protective factors of stress helps better predict birthweight of the human newborns (Brotnow et al., 2015). Another study did a meta-analysis on 88 prospective studies about maternal prenatal stress and infant birthweight between 1970 and 2012 (Bussières et al., 2015). It found a significant but weak negative correlation between maternal prenatal stress and birthweight (Bussières et al., 2015). A review article on human studies also found that both male and female offspring are susceptible to prenatal stress (Van den Bergh et al., 2016). A literature review article studied prenatal stress and brain development in both human and rodent models (Lindsay et al., 2018). The paper found significant heterogeneity in the interactions of prenatal nutrition intake and prenatal stress on brain development of the offspring, due to different study designs, standards in measuring nutrition, assessment of brain development, the prenatal stressor, and sex differences (Lindsay et al., 2018).
Overall, for prenatal maternal stress, past research showed a heterogeneity in relation to food and water consumption of the mothers, body weight of both mothers and the offspring, brain sizes and behaviors of the offspring in stress versus non-stress groups. The severity of stressors, types of rodent, and types of feed etc. could all be moderators affecting the various responses among rodent models.
The current study focused on the influences of prenatal maternal stress and its effects on food, water intake, and body weight gain of the pregnant mice. It is designed to ensure that behavioral effects are not driven by changes in maternal nutritional intake induced by stress, the proposed experiment examines daily changes in food and water consumption and rate of weight gain of pregnant mice during breeding and gestation under stress vs. non-stress condition. Due to the heterogeneity of findings, it is critical to determine whether these aspects are impacted for each particular studied stress paradigm, to establish whether nutritional aspects might be mediating the effects of stress on offspring behavior. Offspring in the control diet-stress condition are expected to show more autism-associated behaviors than the DHA-stress group. We hypothesized that no significant difference in body weight gain, food intake, and water intake would be seen in mice during pregnancy between the stressed and non-stressed conditions. Therefore, the behavioral changes among the offspring are not associated with significant differences in food, water consumption, and weight changes among these groups.
2. Experimental Procedures
2.1. Animals
All the experimental male mice with a C57BL/6J genotype were obtained from The Jackson Laboratory (Bar Harbor, ME). These male mice mated with the female mice with also a C57BL/6J background. The lab room where the mice were kept in has a stable temperature of 21 °C and a 12:12 light: dark cycle with light turned on at 8:00 AM every day. Male and female mice were housed separately in 30 cm × 16 cm × 13 cm hanging Plexiglas cages with aspen wood shaving bedding. These cages served as the house of the mice. Before mating, each male was housed individually in cages and four females shared the same cage. Food and water were available for them the whole time and was changed once a week.
2.2. Breeding
Each single male was moved into the standard cage with two single females for breeding after all experimental mice reach puberty. Researchers checked for vaginal plugs for females every day before 7 AM. A vaginal plug indicates the female mouse has copulated and therefore is likely pregnant. All the dams (n=22) were housed separately since the 8th day of their pregnancy.
2.3 Chronic variable stress paradigm
Ten dams were randomly assigned to the stress protocol starting the 6th day of pregnancy until the birth for all mice. The stressed condition was implemented by constant light exposure (36 hours), exposure to fox urine (1 hour), overnight exposure to novel objects (marbles) in the cage, restraints (10 minutes), overnight exposure to novel noise, and multiple cage changes during the light cycle. One stressor was presented per day over a 7-day period and this sequence was repeated 3 times. To be more specific, the fox urine used in the experiment was obtained from Wildlife Research Center and one ML of it was placed in the corner of the mouse’s house for an hour. After one hour, experimenter switched the mouse back to a clean cage. For the restraint process, the experimenter put the mice in a restraint Plexiglas tube for 10 minutes. There are holes along the entire tube, as well as on both ends. However, the mice would not be able to move out of the tube. In addition, for the novel noise, the dams were also exposed to moderately loud static noise created by a boombox on radio setting on a non-station signal in a room separate from all other mice. Dams have their cage changed by the experimenter three times in one day, approximately every 4 hours. The stressed groups continued experiencing stress until their offspring were born. The controlled groups (n=12) were under normal, non-stressed condition in their individual houses.
All dams were all fed AIN-93G (Research Diets, D10012G), the control diet for throughout the entire experiment. In total, the experiment had two groups of mice. The stressed group has 10 mice and the control group has 12 mice. They were under non-stressed/controlled diet (control group) and stressed/controlled diet (stressed group) condition. Three main elements were measured in this experiment: weight changes, food consumption, and water consumption. These data were obtained by weighing each dam, her food, and her water on a scale every morning at 8 AM during the whole period of pregnancy, which was roughly 18 days. Their weight change, food and water consumption were recorded. Two-sample t-tests were performed with Minitab 18 to compare the body weight, food and water consumption between the control group and the stressed group since the day observed the plug until the day of delivery.
3. Result
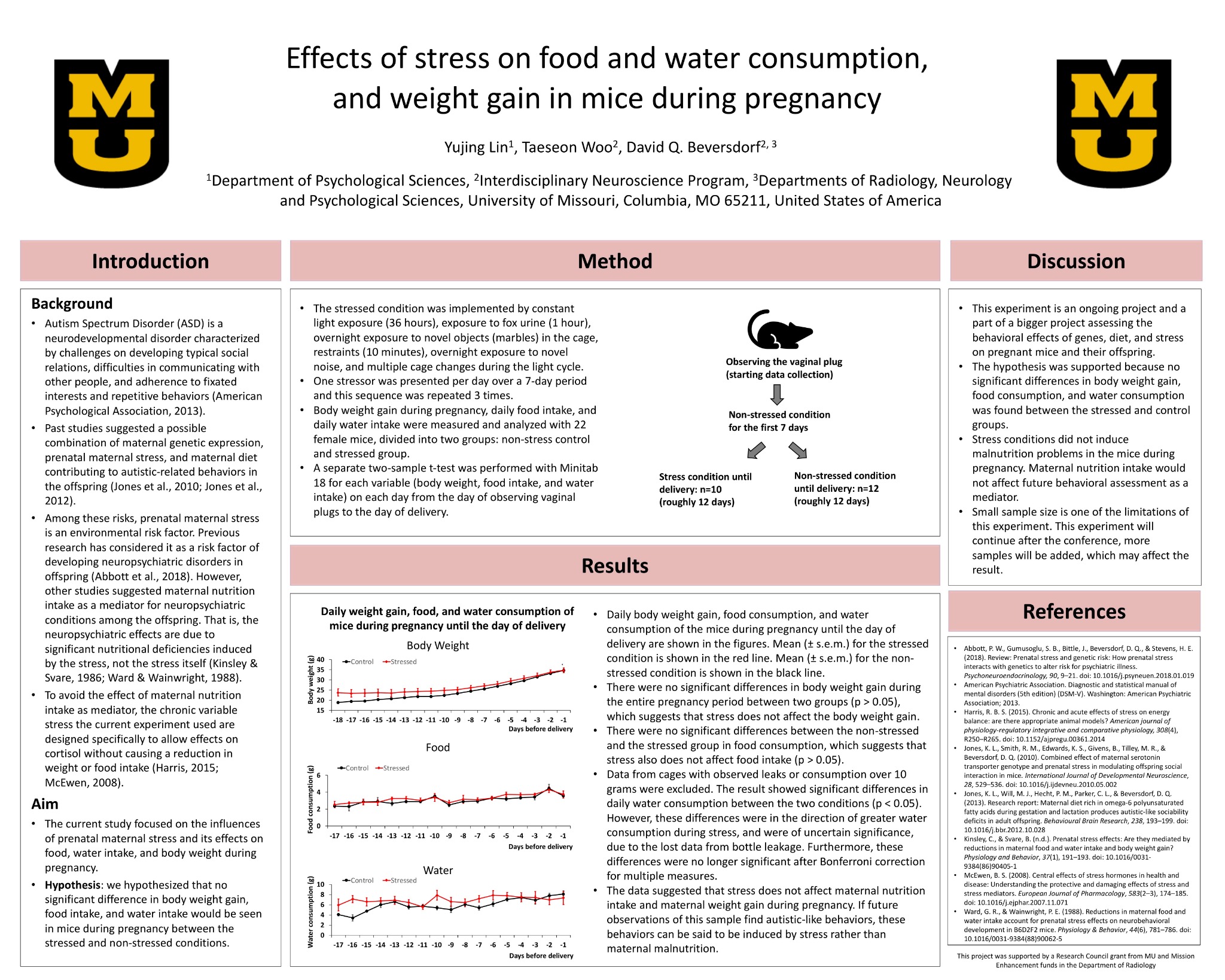
Body weight gain during pregnancy, daily food consumption, and daily water consumption were measured and analyzed with 22 female mice, divided into two groups: non-stress control (n=12) and stressed group (n=10). A separate two-sample t-test was performed for each variable (body weight, food consumption, and water consumption) on each day from the day of observing plugs to the day of delivery, in order to compare the differences between the non-stress and the stress dams. The result revealed no significant differences in body weight gain, food and water consumption between the two groups.
3.1 Body Weight
The average body weight gains of all the non-stress dams and all the stress dams on each day were calculated and depicted in Figure 1. All the mice were in the non-stressed, control diet condition until the 7th day after first observing the vaginal plugs. Then, 10 dams were moved to the stress room and experienced the stress protocol while the remaining 12 dams stayed in the non-stress condition. There were no significant differences in body weight gain during the entire pregnancy period between two groups (p > 0.05). The result suggested that stress does not affect body weight gain of dams during pregnancy, because there were no significant differences between the two conditions.
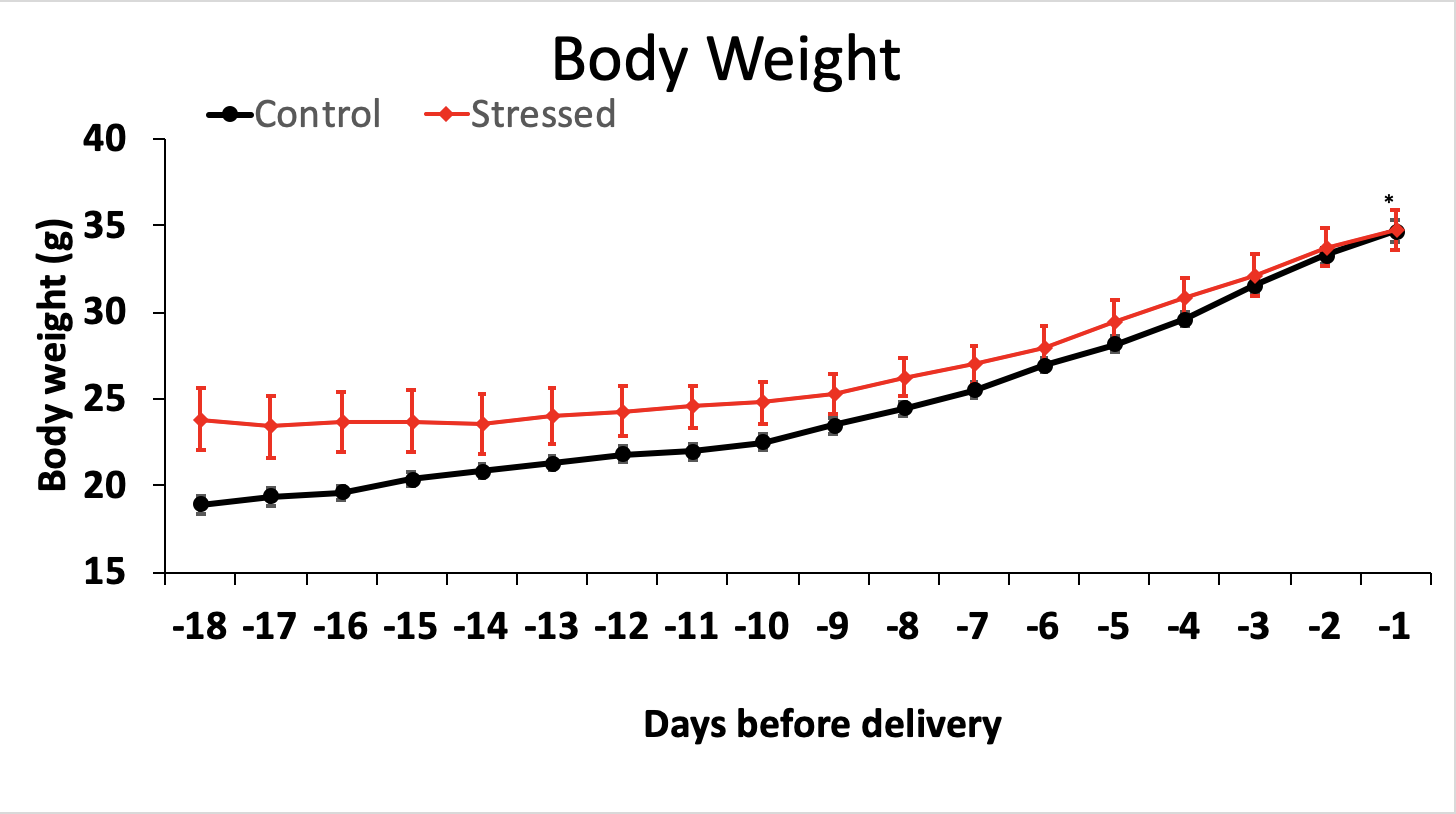
Figure 1 Daily weight gain of the mice during pregnancy until the day of delivery. Mean (± s.e.m.) daily weight gain for the stressed/regular diet condition is shown in the red line. Mean (± s.e.m.) daily weight gain for the non-stressed/regular diet condition is shown in the black line (p > 0.05).
3.2 Food Consumption
The average daily food consumption of all the dams were calculated and shown in Figure 2. The stressed/normal-diet dams consumed 3.256 grams of food and the non-stressed/normal-diet dams consumed 3.058 grams of food on average every day. There were no significant differences between the non-stressed and the stressed group in food consumption (p > 0.05).

Figure 2 Daily food consumption of the mice during pregnancy until the day of delivery. Mean (± s.e.m.) daily food consumption for the stressed/regular diet condition is shown in the red line. Mean (± s.e.m.) daily food consumption for the non-stressed/regular diet condition is shown in the black line (p > 0.05).
3.3 Water Consumption
The average daily water consumption of all the dams were calculated and depicted in Figure 3. The stressed/normal-diet dams drank 6.939 grams of water and the controlled dams drank 6.165 grams of water on average every day. However, due to leaks in several of the water bottles during some of the days, the data is limited to those without any leaks, thus decreasing the sample size. Data from cages with observed leaks or consumption over 10 grams were excluded. The result showed significant differences in daily water consumption between the two groups on day 16, 10, and 7 before delivery (p < 0.05). However, these differences were in the direction of greater water consumption during stress, and were of uncertain significance, due to the lost data from bottle leakage. Furthermore, these differences were no longer significant after Bonferroni correction for multiple measures.

Figure 3 Daily water consumption of the mice during pregnancy until the day of delivery. Mean (± s.e.m.) daily water consumption for the stressed/regular diet condition is shown in the red line. Mean (± s.e.m.) daily water consumption for the non-stressed/regular diet condition is shown in the black line (p < 0.05).
4. Discussion
The aim of this study was to ensure the stress protocol does not affect nutrition intake of the dams during pregnancy. Previous research has shown that prenatal stress may lead to maternal malnutrition, and nutrition intake may affect the likelihood of observing autistic behaviors among the offspring (Kinsley & Svare, 1986; Ward & Wainwright, 1988). This experiment was designed to ensure that future observation of behavioral changes was induced by stress itself rather than a lack of nutrition.
Our results support the hypothesis that no significant differences in body weight gain, food consumption, and water consumption were found between the stressed and the control groups. This suggested that the chronic variable stress the current experiment used did not induce malnutrition problems in the mice during pregnancy. Maternal nutrition intake and appropriate weight gain during pregnancy would not affect future behavioral assessment as a mediator. Therefore, this stress protocol is specifically to allows effects on cortisol, a hormone secreted by the adrenal gland in response to stress, without causing a reduction in weight or nutrition intake.
Our findings differ from the findings of Kinsley & Svare (1986) and Ward & Wainwright (1998), where such nutritional effects were observed with stress. However, the stress protocols used in the previous studies are different from the current study. One previous study used two daily one-hour sessions of restraint stress in a folded wire mesh screen (20 x 20 cm) for 6 consecutive days from the 12th day to the 17th day of pregnancy (Ward & Wainwright, 1998). The other study used heat and restraint stress, which involved putting each female into a 3.25 x 1.124 in Plexiglas restraint tube over two 150-watt flood lights (Kinsley & Svare, 1986). The temperature of the restraint tube was approximately 38 °C (Kinsley & Svare, 1986). The restraint stress lasted from day 13 of gestation through day 18 and there were three 30-minute stress sessions each day (Kinsley & Svare, 1986). Both experiments found decreased rates of body weight gain and decreased water and food intake among the stressed dams comparing to the non-stressed dams. They suggested that stress protocols these experiments used would lead to maternal malnutrition, which could be a mediator in behavioral effects of the offspring.
The stress protocol the present experiment used was chronic variable stress paradigm. It consisted of once daily exposure to one each of a rotating set of stressors, including constant light exposure, exposure to fox urine scent, overnight exposure to novel objects in the cage, restraints, overnight exposure to novel noise, and multiple cage changes during the light cycle. Comparing to the stress protocols that previous experiments used, the chronic variable stress paradigm was less severe in the current study. For example, the restraint in this study was 10 minutes, compared to the one-hour stress session in Ward & Wainwright (1998) and the 30-minute stress session in Kinsley & Svare (1986). Also, the current experiment only presented one stressor per day. Previous studies repeated their stress sessions for more than once in a day (Kinsley & Svare, 1986; Ward & Wainwright, 1998). These differences may be the reason why the stress protocol of the current study did not lead to significant differences between the stress and control groups, which means that the possibility of maternal malnutrition causing autistic like behaviors among the offspring is not a factor in this project.
However, there are limitations of the study. The sample size is small. There were only 22 dams in total, with 10 in the stressed group and 12 in the control group. Since this experiment is a part of a bigger, ongoing project, more data on mice will be collected. By that time, the sample size will be much larger.
Another limitation of the experiment is the water leaking issue throughout the experiment. More than half of the data from water consumption was taken out due to inaccuracy. Future study should use better water bottles to prevent the leaking issue to ensure there is enough data to analyze for daily water consumption.
A future direction for this study is adding diet as another independent variable. Both stress and diet are environmental factors that affect behaviors among the offspring. Specific factors in the diet may also affect the body weights and nutrition intake of the mothers as well as the brain health and the development of diseases in the offspring (Sun et al., 2017). One of the factors in diet is the intake of polyunsaturated fatty acid, such as DHA. DHA is an important structural constituent of membranes in the brain (Lauritzen et al., 2016). It is critical for fetal brain development especially during the last trimester of pregnancy and essential to the functioning and development of the central nervous system (Jones et al., 2012; Ahmad & Salem, 2002).
Past experiments have suggested a mitigation effect of DHA against prenatal maternal stress (Takeuchi et al., 2002). A diet supplemented with DHA may help reduce the possibilities of having psychiatric disorders (Lauritzen et al., 2016). DHA deficiency may be correlated with the development of psychiatric disorders or chronic diseases (Matsui et al., 2018; Simopoulos, 2002). With 42 male Sprague Dawley rats, past research studied potential neuroprotective effects of n-3 Polyunsaturated fatty acids (n-3PUFAs) for mild physiological stress and anxiety behaviors (Appleton et al., 2014). The experiment used deoxycorticosterone acetate (DOCA) administration to induce gradual physiological stress to the mice. The DOCA-treated mice were found to be consuming significantly more water than the control mice. They also showed a significant decrease in body weight compared to the mice that did not receive DOCA. The results demonstrated a disruption to the renin-angiotensin-aldosterone system by the mild physiological stress. The stress also had an impact on anxiety behaviors in the mice. However, the study did not find any effects of n-3PUFAs diet. Changes in food and water consumption and body weight of the mice were not significantly different between control diet versus a high n-3PUFAs experimental diet in mice that received DOCA (Appleton et al., 2014). Another study used Long Evans female rats received from Charles River to analyze the effect of a DHA-deficient diet on the development of body, brain, and neurons (Ahmad & Salem, 2002). The mice in the non-DHA diet group (the control group) had a significantly lower body weight, but no significantly lower brain weight compared to the DHA diet group (Ahmad & Salem, 2002). The study also found a reduction in neuron sizes in brains of the offspring given a DHA-deficient diet (Ahmad & Salem, 2002).
In short, previous studies fail to provide homogeneous evidence that there are significant differences in changes of food, water intake, weight, and brain sizes associated with DHA diet during pregnancy versus non-DHA diet.
For future studies, researchers can examine four different groups: non-stressed/control diet, non-stressed/DHA diet, stressed/control diet, and stressed/DHA diet. With this, researchers can study whether DHA diet combined with prenatal stress have an effect on nutrition intake and body weight gains of dams.
Further studies will also include behavioral assessments of the offspring and compared the brain sizes of the pups from stressed mothers versus non-stressed mothers.
The result of this experiment can be used in future studies of prenatal stress. If other researchers want to learn about the relationship between prenatal stress and autistic behaviors among the offspring using an animal model, the stress protocol used in this experiment can ensure behavioral effects and psychiatric disorders are induced by the stress itself rather than other factors like maternal malnutrition.
Reference
Abbott, P. W., Gumusoglu, S. B., Bittle, J., Beversdorf, D. Q., & Stevens, H. E. (2018). Review: Prenatal stress and genetic risk: How prenatal stress interacts with genetics to alter risk for psychiatric illness. Psychoneuroendocrinology, 90, 9–21. https://doi.org/10.1016/j.psyneuen.2018.01.019
Ahmad, A., Moriguchi, T., & Salem, J. N. (2002). Original article: Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatric Neurology, 26, 210–218. https://doi.org/10.1016/S0887-8994(01)00383-6
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th edition) (DSM-V). Washington: American Psychiatric Association; 2013.
Appleton, K. M., Grippo, A. J., Beltz, T. G., & Johnson, A. K. (2015). Consumption of a high n-3 polyunsaturated fatty acid diet during gradual mild physiological stress in rats. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA), 95, 11–18. https://doi.org/10.1016/j.plefa.2014.11.010
Beversdorf, D., Manning, S., Hillier, A., Anderson, S., Nordgren, R., Walters, S., Nagaraja, H., Cooley, W., Gaelic, S., & Bauman, M. (2005). Timing of Prenatal Stressors and Autism. Journal of Autism & Developmental Disorders, 35(4), 471–478. https://doi.org/10.1007/s10803-005-5037-8
Brotnow, L., Reiss, D., Stover, C. S., Ganiban, J., Leve, L. D., Neiderhiser, J. M., & Stevens, H. E. (2015). Expectant mothers maximizing opportunities: Maternal characteristics moderate multifactorial prenatal stress in the prediction of birth weight in a sample of children adopted at birth. PLoS ONE, 10(11). Retrieved from http://proxy.mul.missouri.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2015-52913-001&site=eds-live&scope=site DOI: 10.1037/t21264-000
Bussières, E.-L., Tarabulsy, G. M., Pearson, J., Tessier, R., Forest, J.-C., & Giguère, Y. (2015). Maternal prenatal stress and infant birth weight and gestational age: A meta-analysis of prospective studies. Developmental Review, 36, 179–199. https://doi.org/10.1016/j.dr.2015.04.001
Cabrera R.J., Rodríguez-Echandía E.L., Jatuff A.S.G., & Fóscolo M. (1999). Effects of prenatal exposure to a mild chronic variable stress on body weight, preweaning mortality and rat behavior. Brazilian Journal of Medical and Biological Research, Vol 32, Iss 10, Pp 1229-1237, (10). Retrieved from http://proxy.mul.missouri.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=edsdoj&AN=edsdoj.292387b2de7e46eab779f62d0eeb4912&site=eds-live&scope=site
Carlson, S. E., Gajewski, B. J., Alhayek, S., Colombo, J., Kerling, E. H., & Gustafson, K. M. (2018). Dose–response relationship between docosahexaenoic acid (DHA) intake and lower rates of early preterm birth, low birth weight and very low birth weight. Prostaglandins, Leukotrienes and Essential Fatty Acids, 138, 1–5.https://doi.org/10.1016/j.plefa.2018.09.002
Gartland, N., O, C. D. B., Lawton, R., & Bristow, M. (2014). Exploring day-to-day dynamics of daily stressor appraisals, physical symptoms and the cortisol awakening response. Psychoneuroendocrinology, 50, 130–138. https://doi.org/10.1016/j.psyneuen.2014.08.006
Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet 1998;352(August (9129)):707–8. http://dx.doi.org/10.1177/2167702618811079
Gur, T. L., Shay, L., Palkar, A. V., Fisher, S., Varaljay, V. A., Dowd, S., & Bailey, M. T. (2017). Named Series: Brain, Behavior, Immunity and the Microbiome: Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behavior and Immunity, 64, 50–58. https://doi.org/10.1016/j.bbi.2016.12.021
Harris, R. B. S. (2015). Chronic and acute effects of stress on energy balance: are there appropriate animal models? American journal of physiology-regulatory integrative and comparative physiology, 308(4), R250–R265. https://doi.org/10.1152/ajpregu.00361.2014
Jones, K. L., Smith, R. M., Edwards, K. S., Givens, B., Tilley, M. R., & Beversdorf, D. Q. (2010). Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction in mice. International Journal of Developmental Neuroscience, 28, 529–536. https://doi.org/10.1016/j.ijdevneu.2010.05.002
Jones, K. L., Will, M. J., Hecht, P. M., Parker, C. L., & Beversdorf, D. Q. (2013). Research report: Maternal diet rich in omega-6 polyunsaturated fatty acids during gestation and lactation produces autistic-like sociability deficits in adult offspring. Behavioural Brain Research, 238, 193–199. https://doi.org/10.1016/j.bbr.2012.10.028
Kinsley, C., & Svare, B. (n.d.). Prenatal stress effects: Are they mediated by reductions in maternal food and water intake and body weight gain? Physiology and Behavior, 37(1), 191–193. https://doi.org/10.1016/0031-9384(86)90405-1
Lauritzen, L., Brambilla, P., Mazzocchi, A., Harsløf, L. B. S., Ciappolino, V., & Agostoni, C. (2016). DHA Effects in Brain Development and Function. Nutrients, 8(1). https://doi.org/10.3390/nu8010006
Lindsay, K. L., Buss, C., Wadhwa, P. D., & Entringer, S. (2018). Review: The Interplay Between Nutrition and Stress in Pregnancy: Implications for Fetal Programming of Brain Development. Biological Psychiatry. https://doi.org/10.1016/j.biopsych.2018.06.021
Lyall, K., Croen, L., Daniels, J., Fallin, M. D., Ladd-Acosta, C., Lee, B. K., … Newschaffer, C. (2017). The Changing Epidemiology of Autism Spectrum Disorders. Annual Review Of Public Health, 38, 81–102. https://doi.org/10.1146/annurev-publhealth-031816-044318
Matsui, F., Hecht, P., Yoshimoto, K., Watanabe, Y., Morimoto, M., Fritsche, K., Will, M., Beversdorf, D. (2018). Research Article: DHA Mitigates Autistic Behaviors Accompanied by Dopaminergic Change in a Gene/Prenatal Stress Mouse Model. Neuroscience, 371, 407–419. https://doi.org/10.1016/j.neuroscience.2017.12.029
McEwen, B. S. (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583(2–3), 174–185. https://doi.org/10.1016/j.ejphar.2007.11.071
Ramakrishnan, U., Stinger, A., DiGirolamo, A. M., Martorell, R., Neufeld, L. M., Rivera, J. A., Wang, M. (2015). Prenatal docosahexaenoic acid supplementation and offspring development at 18 months: Randomized controlled trial. PLoSONE, 10(8). https://doi.org/10.1371/journal.pone.0120065
Rhees, R. W., & Fleming, D. E. (n.d.). Effects of malnutrition, maternal stress, or ACTH injections during pregnancy on sexual behavior of male offspring. Physiology and Behavior, 27(5), 879–882. https://doi.org/10.1016/0031-9384(81)90057-3
Romero-Gonzalez, B., Caparros-Gonzalez, R. A., Gonzalez-Perez, R., Delgado-Puertas, P., & Peralta-Ramirez, M. I. (2018). Newborn infants’ hair cortisol levels reflect chronic maternal stress during pregnancy. PLoS ONE, 1. Retrieved from http://proxy.mul.missouri.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=edb&AN=130568190&site=eds-live&scope=site
Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine and Pharmacotherapy 2002; 56(October (8)):365–79. DOI: 10.1016/S0753-3322(02)00253-6
Sun, G. Y., Simonyi, A., Fritsche, K. L., Chuang, D. Y., Hannink, M., Gu, Z., Beversdorf, D. Q. (2017). Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA). https://doi.org/10.1016/j.plefa.2017.03.006
Takeuchi, T., Iwanaga, M., & Harada, E. (2003). Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Research, 964(1), 136–143. https://doi.org/10.1016/S0006-8993(02)04113-6
Van den Bergh, B. R. H., Schwab, M., van den Heuvel, M. I., Lahti, M., Räikkönen, K., Braeken, M., … King, S. (n.d.). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience and Biobehavioral Reviews. https://doi.org/10.1016/j.neubiorev.2017.07.003
Ward, A. J. (1990). A comparison and analysis of the presence of family problems during pregnancy of mothers of “autistic” children and mothers of normal children. Child Psychiatry and Human Development, 20(4), 279–288. https://doi.org/10.1007/BF00706020
Ward, G. R., & Wainwright, P. E. (1988). Reductions in maternal food and water intake account for prenatal stress effects on neurobehavioral development in B6D2F2 mice. Physiology & Behavior, 44(6), 781–786. https://doi-org.proxy.mul.missouri.edu/10.1016/0031-9384(88)90062-5
Wong, J. D., Seltzer, M. M., Greenberg, J. S., Hong, J., Almeida, D. M., & Coe, C. L. (2012). Stressful life events and daily stressors affect awakening cortisol level in midlife mothers of individuals with autism spectrum disorders. Aging & Mental Health, 16(8), 939–949. https://doi.org/10.1080/13607863.2012.688191